Find the Right Dilution Factor for Any Solution in Seconds
The Dilution Factor Calculator helps you accurately calculate serial dilutions, mg/mL concentrations, and microbiology solutions. Quickly determine the right volumes and dilution factors for scientific, industrial, and laboratory applications.
Dilution Visualization
Dilution Visualization
| Tube | Concentration | Transfer Volume | Diluent Volume |
|---|
Common Dilution Reference Guide:
1:10 Dilution
10x dilution factor
1:100 Dilution
100x dilution factor
1:1000 Dilution
1000x dilution factor
1:2 Dilution
2x dilution factor
The Dilution Factor Calculator is a powerful tool designed for laboratory professionals, scientists, and students who need to perform accurate dilution calculations. Whether you are working in chemistry, biology, pharmaceuticals, or environmental science, this tool helps you determine the exact volumes and concentrations required for your solutions.
What is Dilution?
Dilution is the process of reducing the concentration of a solute in a solution by adding more solvent. This is commonly done to prepare reagents, adjust sample concentrations, or create stepwise dilutions in laboratory experiments.
How to Use the Dilution Factor Calculator
This calculator offers different calculation modes depending on your needs. Below are the available modes and how to use each one:
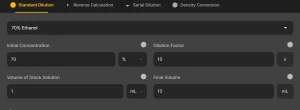
1. Standard Dilution Calculation
Use this mode when you know the initial concentration and want to dilute it to a lower concentration.
Input Fields
- Initial Concentration (C₁): The starting concentration of the solution. Enter values in molarity (M), percentage (%), or mass/volume (mg/mL, µg/mL, etc.).
- Dilution Factor: The ratio by which the solution should be diluted. A dilution factor of 10 means a 1:10 dilution (1 part stock + 9 parts solvent).
- Initial Volume (V₁): The volume of the stock solution you plan to dilute.
- Final Volume (V₂): The total volume of the diluted solution after adding the solvent.
Example
If you have 1M hydrochloric acid (HCl) and need to prepare 100 mL of a 0.1M solution, enter:
- Initial Concentration: 1M
- Dilution Factor: 10
- Initial Volume: 10 mL
- Final Volume: 100 mL
The calculator will tell you that you need to mix 10 mL of stock solution with 90 mL of water to achieve the desired dilution.
For more advanced dilution calculations, you can check our solution dilution calculator.
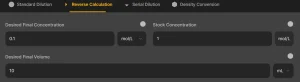
2. Reverse Dilution Calculation
This mode helps determine how much of the stock solution is needed to prepare a specific volume and concentration.
Input Fields
- Desired Final Concentration (C₂): The concentration you want to achieve.
- Stock Solution Concentration (C₁): The original concentration of the solution.
- Final Volume (V₂): The total volume of the new diluted solution.
Example
You need 200 mL of a 0.5M sodium chloride (NaCl) solution, and you have 5M stock solution. Enter:
- Final Concentration: 0.5M
- Stock Concentration: 5M
- Final Volume: 200 mL
The calculator will show that you need to take 20 mL of the 5M solution and add 180 mL of water to make the final dilution.
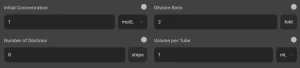
3. Serial Dilution Calculation
Serial dilution is used when multiple stepwise dilutions are required, such as in microbial cultures, enzyme assays, and chemical reactions.
Input Fields
- Initial Concentration (C₁): The starting concentration of the solution.
- Dilution Ratio: The factor by which the solution is diluted in each step (e.g., 2 for a 1:2 dilution).
- Number of Dilutions: The number of times the dilution is repeated.
- Volume per Tube: The volume in each test tube.
Example
If you are performing a 1:10 serial dilution starting with 1 mg/mL of a protein solution over 5 dilution steps, enter:
- Initial Concentration: 1 mg/mL
- Dilution Ratio: 10
- Number of Dilutions: 5
- Volume per Tube: 1 mL
The calculator will generate a table showing the concentrations at each step.
For more detailed dilution ratio calculations, visit our Dilution ratio calculation.
4. Density Conversion Calculation
This mode is useful when working with solutions where concentration is given as a percentage, and you need to convert between weight/volume (% w/v) and mass/volume (mg/mL).
Input Fields
- Weight/Volume (%): The percentage concentration of the solution.
- Solution Density: The density of the solution (optional for precise conversions).
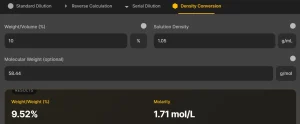
Example
If you have 10% glucose solution (w/v) and want to find the concentration in mg/mL, enter:
- Weight/Volume: 10%
- Density: Assumed as 1 g/mL
The result will show that 10% w/v = 100 mg/mL.
For more advanced dilution calculations for oligonucleotide (DNA, RNA) dilutions, check our Oligo dilution calculator.
Understand and Use “Common Laboratory Solution” Feature
The Common Laboratory Solution feature in the Dilution Factor Calculator simplifies the process of setting up dilution calculations by providing pre-configured solutions with standardized concentrations. This tool is essential for scientists, researchers, and laboratory professionals who regularly work with commonly used solutions in chemistry, microbiology, molecular biology, and pharmaceutical sciences.
What It Is
This feature is a pre-set solution library that allows users to select from a list of commonly used laboratory solutions without manually entering their initial concentrations and units. When you choose a solution from the dropdown, the calculator automatically fills in the appropriate values, reducing errors and saving time.
How to Use It
- Click on the “Select Common Laboratory Solution” dropdown in the Standard Dilution tab.
- Browse through the list of available laboratory solutions.
- Select your desired solution from the menu.
- The calculator will automatically populate the initial concentration and units.
- Enter the remaining dilution parameters, such as final volume or dilution factor, and proceed with the calculation.
Available Solutions
The Common Laboratory Solution feature includes a carefully curated list of frequently used lab solutions across biological, chemical, and industrial applications.
- 1M Hydrochloric Acid (HCl) – A strong acid commonly used for pH adjustments, buffer preparation, and reagent-based reactions.
- 0.1M Sodium Hydroxide (NaOH) – A base solution widely used in titrations, chemical neutralization, and biochemical research.
- 10% Formalin – A formaldehyde-based solution frequently used as a tissue fixative in histology and pathology labs.
- 5% Acetic Acid – A mild acid used in biochemical research, histology staining, and acid-base reactions.
- 10 mg/mL BSA (Bovine Serum Albumin) – A standard protein solution used in protein assays, Western blotting, and ELISA procedures.
- 70% Ethanol – A commonly used antiseptic, disinfectant, and preservative in laboratories.
- 1 mg/mL Protein – A standard protein concentration used in biochemical assays, enzyme studies, and molecular biology research.
- 1 µg/mL DNA – A standard dilution for DNA stock solutions used in PCR, qPCR, sequencing, and molecular biology applications.
Benefits of Using the Common Laboratory Solution Feature
- Accuracy – Ensures that commonly used solutions have the correct standardized concentrations, minimizing calculation errors.
- Efficiency – Saves time by automating the input process, eliminating the need for manual concentration entries.
- Consistency – Helps maintain uniform solution preparation across multiple experiments, research studies, and industrial processes.
- Educational Value – Serves as a reference tool for students, new laboratory personnel, and researchers who may need guidance on standard laboratory solution concentrations.
This feature is particularly useful for professionals working with serial dilutions, buffer preparations, microbial cultures, or pharmaceutical formulations.
By integrating these pre-configured laboratory solutions, the Dilution Factor Calculator makes scientific workflows faster, more reliable, and error-free.
Common Dilution Factor Related Q&A
What is a dilution factor?
The dilution factor (DF) is the ratio of the final solution volume to the initial solution volume. It is calculated as:
DF = V₂ / V₁
A 1:10 dilution means taking 1 part stock solution and adding 9 parts solvent.
How do I prepare a 1:100 dilution?
To make a 1:100 dilution, take 1 mL of the stock solution and add 99 mL of solvent. This reduces the concentration 100 times.
What is the difference between dilution factor and dilution ratio?
- Dilution Factor (DF) is the total volume divided by the stock volume.
- Dilution Ratio is expressed as 1:X, meaning 1 part solute + X parts solvent.
Why are serial dilutions used in microbiology?
Serial dilutions are used to create progressively lower concentrations of a sample, which helps in counting bacteria, performing enzyme kinetics, and studying chemical reactions.
Can I use this calculator for medical or pharmaceutical dilutions?
Yes, this calculator is useful for IV solution preparation, drug dilutions, and diagnostic test preparation. Always verify calculations with a pharmacist or medical professional.