Serial Dilution Calculator – Optimize Your Lab Dilutions Instantly
Serial Dilution Calculator – Easily calculate 2-fold, 4-fold, CFU, percent, ELISA, and more with precision. Enter your values and get instant dilution steps, transfer volumes, and final concentrations for accurate lab experiments! 🚀
| Tube | Concentration | Transfer Volume | Diluent Volume |
|---|
Direct Dilution Scheme
| Dilution | Concentration | Stock Volume | Diluent Volume |
|---|
1:10 Dilution
10x dilution factor
1:100 Dilution
100x dilution factor
1:1000 Dilution
1000x dilution factor
1:2 Dilution
2x dilution factor
Serial Dilution Guide
Serial dilution is a step-wise dilution technique used to create a series of solutions with decreasing concentrations. This guide explains the different methods and applications of serial dilutions.
Transfer Method vs. Direct Method
Direct Method: In this method, each dilution is prepared directly from the stock solution. This is useful when you need precise concentrations and want to avoid cumulative errors.
Common Applications of Serial Dilutions
- Microbiology: Counting bacteria or other microorganisms by diluting samples to countable levels
- Biochemistry: Creating standard curves for protein or DNA quantification
- Pharmacology: Testing drug efficacy at different concentrations
- Immunology: Antibody titration and ELISA assays
- Cell Biology: Cell counting and viability assays
Tips for Accurate Serial Dilutions
- Use calibrated pipettes and mix thoroughly between each transfer
- Change pipette tips between each transfer to prevent carryover
- Prepare dilutions from lowest to highest concentration to minimize errors
- Include controls and blanks in your experimental design
- For very precise work, prepare each dilution directly from the stock
- Consider the solubility of your compound in the diluent
Common Dilution Factors
The most commonly used dilution factors in laboratory settings are:
2-fold (1:2)
Each step is half the previous concentration
5-fold (1:5)
Each step is 1/5 the previous concentration
10-fold (1:10)
Each step is 1/10 the previous concentration
Log dilutions
Dilutions that follow a logarithmic scale
Calculating Dilution Factor
The dilution factor can be calculated using the following formulas:
Dilution Factor = Final Volume ÷ Initial Volume
or
Dilution Factor = Initial Concentration ÷ Final Concentration
For serial dilutions, the total dilution factor is the product of all individual dilution factors.
Introduction to Serial Dilutions
Serial dilution is a fundamental laboratory technique used to systematically reduce the concentration of a substance in a solution. This method involves transferring a fixed volume of the original solution into a diluent, then repeating the process multiple times to create a dilution series.
It is commonly applied in:
- Microbiology – Counting bacteria or viruses by diluting samples to countable levels.
- Biochemistry – Preparing standard curves for assays such as ELISA and spectrophotometric analyses.
- Pharmacology – Testing drug efficacy at different concentrations.
- Cell Biology – Diluting cell cultures for toxicity and viability studies.
Performing these calculations manually can be time-consuming and error-prone, which is why our Serial Dilution Calculator provides a quick, accurate, and visual solution to streamline the process. Whether you’re a student, researcher, or lab technician, this tool ensures precision in your dilution series, saving time and improving reproducibility.
Why Use a Serial Dilution Calculator?
Key Benefits of Using the Calculator
✔ Eliminates Human Error – Ensures precise calculations and avoids dilution miscalculations.
✔ Saves Time – Automates tedious manual calculations.
✔ Enhances Accuracy – Provides step-by-step dilution instructions for consistency.
✔ Visual Representation – Generates dilution diagrams for easy understanding.
✔ Applicable Across Multiple Disciplines – Suitable for microbiology, chemistry, pharmacology, biotechnology, and environmental science.
This calculator is particularly useful for preparing standard solutions, performing microbial counts, drug titrations, and conducting enzyme kinetics studies.
How to Use the Serial Dilution Calculator
Using our tool is super easy, just follow these steps carefully in order to run a serial dilution calculation.
Step 1: Enter Your Starting Parameters
To generate an accurate dilution plan, input the following values:
- Initial Concentration (C₁): The starting concentration of the stock solution.
- Concentration Unit: Choose from mol/L, %, mg/mL, µg/mL, ng/mL, CFU/mL, etc.
- Dilution Factor (DF): The ratio by which each step reduces the concentration (e.g., 1:2, 1:10, 1:100).
- Number of Dilutions (n): The total number of dilution steps required.
- Volume per Tube (V_f): The final volume of solution in each tube.
- Transfer Volume (Vₜ): The amount of solution moved from one tube to the next.
- Diluent Volume (V_d): The volume of buffer or solvent added to achieve dilution.
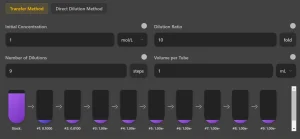
Step 2: View and Interpret Your Results
Once parameters are entered, the calculator automatically:
- Generates a detailed dilution table, including each tube’s concentration, transfer volume, and diluent volume.
- Creates a stepwise visual dilution diagram for better comprehension.
- Provides step-by-step instructions to guide you through the dilution process.
This makes it easy to plan and execute serial dilutions with confidence.
Understanding the Calculator Features
1. Initial Concentration (C₁) and Units
The starting concentration (stock solution) must be entered in the correct unit to ensure accurate calculations. Common units include:
- Molarity (mol/L, M) – Used in chemistry and biochemistry.
- Mass per volume (mg/mL, µg/mL, ng/mL) – Common in pharmacology and microbiology.
- Percentage solutions (%) – Used for buffer and reagent preparations.
- Colony-Forming Units (CFU/mL) – Relevant for bacterial culture counting.
2. Dilution Factor (DF) and Dilution Series
The dilution factor (DF) determines the concentration decrease at each step:
- 1:10 (Ten-fold dilution) – Common in microbiology and environmental science for large reductions.
- 1:2 (Two-fold dilution) – Used in ELISA assays, protein standard curves, and drug response studies.
- 1:5 or 1:4 Dilutions – Intermediate dilutions for achieving finer concentration gradients.
3. Number of Dilution Steps (n)
The more steps, the wider the concentration range:
| Dilution Factor | Steps | Concentration Change |
|---|---|---|
| 1:10 | 3 | 100 → 10 → 1 → 0.1 mg/mL |
| 1:2 | 5 | 100 → 50 → 25 → 12.5 → 6.25 mg/mL |
4. Transfer Volume (Vₜ) and Final Volume (V_f)
- Transfer volume (Vₜ) is how much solution is moved between tubes.
- Final volume (V_f) is the total volume in each dilution tube after adding diluent.
For a 1:5 dilution with a 1 mL final volume:
- Transfer 0.2 mL from the previous tube.
- Add 0.8 mL diluent to reach 1 mL total.
5. Visual Dilution Diagram
Our calculator provides a graphical representation of the dilution series:
✔ Color-coded test tubes to show concentration changes.
✔ Arrows indicating transfer volumes and dilution steps.
✔ Labels displaying exact concentrations at each step.

This feature helps in protocol planning and ensures better visualization before executing the experiment.
6. Dilution Table
The detailed table provides specific information for each dilution step:
1. Tube Number: Identifies each tube in the series
2. Concentration: The final concentration in each tube
3. Transfer Volume: How much to transfer from the previous tube
4. Diluent Volume: How much diluent to add to reach the final volume
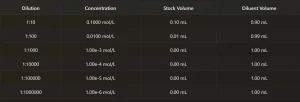
This table serves as your step-by-step guide in the laboratory.
Pro Tip: You might also need to use dilution ratio calculator and general solution dilution finder in your studies, so you are advised to take a look into them as we have them covered.
Tips for Accurate Serial Dilutions
✔ Mix thoroughly after each transfer for homogeneous solutions.
✔ Use fresh pipette tips to avoid contamination.
✔ Work from lowest to highest concentration when using the same pipette.
✔ Label tubes clearly to prevent mix-ups.
✔ Account for pipetting errors and volume losses during transfers.
Common Serial Dilution Patterns
Different dilution patterns serve various scientific applications, depending on the required precision, range, and ease of calculation. Here’s a breakdown of the most commonly used serial dilution methods and their practical uses.
1:10 (Ten-Fold) Dilutions
Widely used in microbiology, environmental science, and pharmaceutical research, ten-fold dilutions allow for rapid concentration reduction across a broad range.
✅ Key Features:
- Each step reduces concentration by 90% (1 part sample + 9 parts diluent).
- Simple to calculate—just move the decimal point.
- Ideal for covering a wide concentration range in fewer steps.
📌 Common Applications:
- Bacterial enumeration (CFU/mL) – Used in plate counting methods for serially diluting high-concentration microbial cultures.
- Turbidity measurements (OD600 in spectrophotometry) – Helps in assessing bacterial growth curves.
- Toxicology and environmental testing – Analyzing contaminants or pollutants at different dilution levels.
🧪 Example Calculation:
A 1:10 dilution series starting with a 100 mg/mL stock solution would yield the following concentrations:
| Step | Concentration (mg/mL) |
|---|---|
| 0 | 100 (Stock Solution) |
| 1 | 10 |
| 2 | 1 |
| 3 | 0.1 |
| 4 | 0.01 |
1:2 (Two-Fold) Dilutions
A two-fold dilution is commonly used in pharmacology, enzyme kinetics, and biochemical assays, where smaller concentration changes provide more resolution in data analysis.
✅ Key Features:
- Each step reduces concentration by 50% (1 part sample + 1 part diluent).
- Provides a greater number of data points within a smaller range.
- Ideal for experiments requiring fine concentration gradients, such as drug-response curves.
📌 Common Applications:
- ELISA and protein quantification assays – Used in standard curve preparation for precise concentration determination.
- Enzyme activity studies – Helps measure reaction kinetics by varying substrate concentrations in small increments.
- Dose-response studies – Used in pharmacology to determine half-maximal effective concentrations (EC50).
🧪 Example Calculation:
A 1:2 dilution series starting with a 50 mg/mL stock solution would yield the following concentrations:
| Step | Concentration (mg/mL) |
|---|---|
| 0 | 50 (Stock Solution) |
| 1 | 25 |
| 2 | 12.5 |
| 3 | 6.25 |
| 4 | 3.125 |
1:4 or 1:5 Dilutions
These dilution factors offer a balanced approach between the large jumps of ten-fold dilutions and the fine precision of two-fold dilutions. If you are interested in dilution factor calculator, we have an advanced dedicated tool for this, take a look here.
✅ Key Features:
- 1:4 dilution – Each step reduces concentration by 75% (1 part sample + 3 parts diluent).
- 1:5 dilution – Each step reduces concentration by 80% (1 part sample + 4 parts diluent).
- Useful for experiments that require gradual concentration reduction without excessive dilution steps.
📌 Common Applications:
- Chemical and pharmacological testing – Determining minimum inhibitory concentration (MIC) of antibiotics or drugs.
- Enzyme inhibition assays – Assessing enzyme activity across multiple concentrations.
- Blood or serum sample dilution – Used in clinical diagnostics and immunoassays.
🧪 Example Calculation:
A 1:5 dilution series starting with a 20 mg/mL stock solution would yield the following concentrations:
| Step | Concentration (mg/mL) |
|---|---|
| 0 | 20 (Stock Solution) |
| 1 | 4 |
| 2 | 0.8 |
| 3 | 0.16 |
| 4 | 0.032 |
Choosing the Right Dilution Pattern
The optimal dilution factor depends on the experimental goals:
| Dilution Factor | Best for | Pros | Cons |
|---|---|---|---|
| 1:10 (Ten-Fold) | Microbial plating, pollutant analysis, toxicology | Covers large concentration ranges in fewer steps | Less precision between dilution points |
| 1:2 (Two-Fold) | Enzyme assays, ELISA, drug testing | More data points for precise analysis | Requires more dilution steps to cover a range |
| 1:4 / 1:5 | Chemical titrations, serum dilutions, enzyme inhibition | Balanced approach between range and precision | More complex calculations than 1:10 or 1:2 |
By selecting the appropriate dilution factor, researchers can optimize their experiments for efficiency, accuracy, and data quality.
Use and Understand Common Laboratory Protocols
Our tool includes pre-set dilution series for commonly used laboratory protocols, ensuring accuracy and efficiency in scientific experiments.
- Protein Standard Curve (2-fold dilution, 1 mg/mL) – Used in protein quantification assays like Bradford and BCA to generate standard curves for unknown protein concentration determination.
- DNA Dilution Series (10-fold dilution, 100 ng/µL) – Essential for PCR, qPCR, and NGS, ensuring DNA samples are diluted to the correct working concentrations.
- Molar Concentration Series (10-fold dilution, 1M solution) – Used for buffer preparation, enzyme reactions, and chemical titrations, ensuring precise molar concentration adjustments.
- Cell Counting Dilution (2-fold dilution, 1000 cells/µL) – Applied in cell culture and microbiology to prepare accurate cell densities for plating, viability assays, and cytometry.
- Antibiotic Sensitivity Test (3-fold dilution, 100 µg/mL) – Used in MIC (Minimum Inhibitory Concentration) assays to evaluate bacterial resistance by testing antibiotics at decreasing concentrations.
- Buffer Concentration Series (2-fold dilution, 10% buffer) – Helps in titrating buffer strength for pH adjustments, biochemical assays, and maintaining stable experimental conditions.